I. Introduction: The Equation of Safety
In the high-stakes environment of modern healthcare, the margin for error is microscopic. Healthcare-Associated Infections (HAIs) remain a formidable adversary, costing the US healthcare system alone billions of dollars annually and affecting hundreds of thousands of patients in Europe.
While systemic protocols often focus on broad environmental hygiene, the most common invasive procedure in medicine—the injection—remains a critical frontline in infection prevention.
To the untrained eye, safety seems guaranteed by the wrapper. However, infection prevention is not a single product; it is an equation. Safety = (Sterile Integrity of the Device) × (Aseptic Technique of the User)
If either variable in this equation is zero, the result is failure.
This article explores the symbiotic relationship between the “hardware”—the sterile disposable needle—and the “software”—the aseptic technique. We will dismantle the misconception that these are separate concerns and demonstrate why high-quality manufacturing and rigorous clinical behavior must align to protect patient lives.
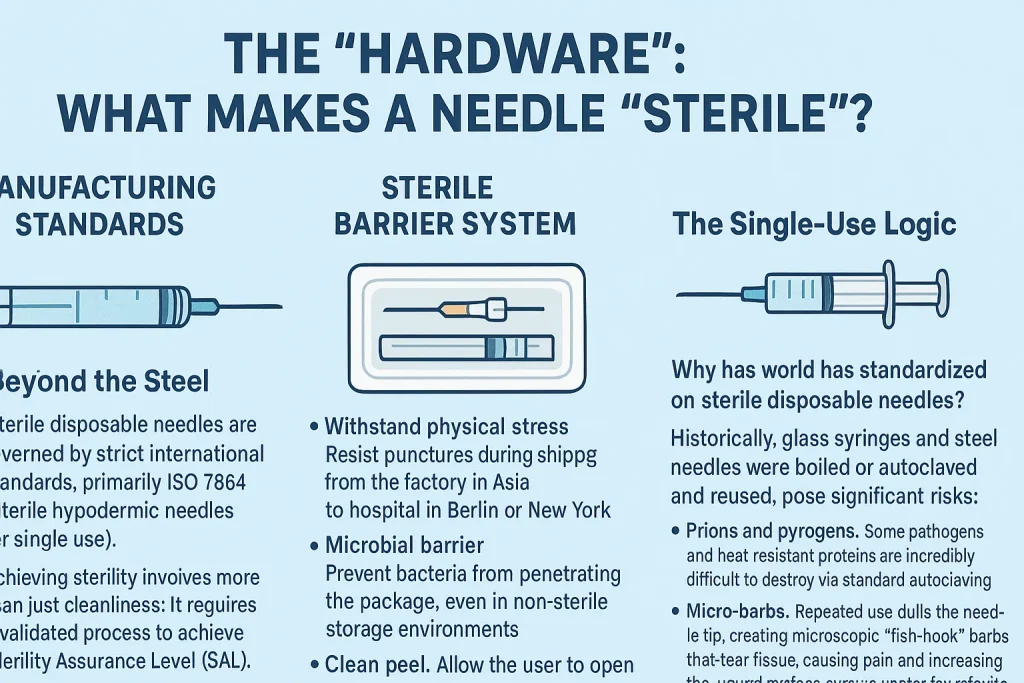
II. The “Hardware”: What Makes a Needle “Sterile”?
A needle is not merely a sharp tube; it is a medical device subject to rigorous engineering standards. When a clinician picks up a package, they are relying on a complex industrial process designed to eliminate every viable microorganism.
Manufacturing Standards: Beyond the Steel
The production of sterile disposable needles is governed by strict international standards, primarily ISO 7864 (Sterile hypodermic needles for single use).
Achieving sterility involves more than just cleanliness; it requires a validated process to achieve a Sterility Assurance Level (SAL) of 10⁻⁶, meaning there is less than a one-in-a-million chance of a viable microorganism remaining on the device.
Table 1: Common Sterilization Methods for Medical Needles
| Feature | Ethylene Oxide (EtO) | Gamma Irradiation |
|---|---|---|
| Mechanism | Chemical gas penetration that disrupts DNA/RNA. | High-energy photons that break chemical bonds in pathogens. |
| Packaging Suitability | Requires breathable packaging (e.g., medical-grade paper) to allow gas ingress/egress. | Compatible with airtight foil or plastic seals. |
| Vantagens | Gentle on materials; ideal for complex plastics. | No chemical residue; immediate sterility; high penetration. |
| Consideration | Requires degassing time to remove residuals. | Can cause discoloration or brittleness in certain polymers if not managed. |
The Sterile Barrier System
The unsung hero of needle safety is the packaging. A needle is only as sterile as its seal. High-quality manufacturers invest heavily in the Sterile Barrier System (SBS). This packaging must:
- Withstand Physical Stress: Resist punctures during shipping from the factory in Asia to a hospital in Berlin or New York.
- Microbial Barrier: Prevent bacteria from penetrating the package, even in non-sterile storage environments.
- Clean Peel: Allow the user to open the pack without tearing, which creates paper dust (particulate contamination) or causes the needle to bounce onto a non-sterile surface.
The Single-Use Logic
Why has the world standardized on sterile disposable needles? Historically, glass syringes and steel needles were boiled or autoclaved and reused. This posed significant risks:
- Prions and Pyrogens: Some pathogens and heat-resistant proteins are incredibly difficult to destroy via standard autoclaving.
- Micro-Barbs: Repeated use dulls the needle tip, creating microscopic “fish-hook” barbs that tear tissue, causing pain and increasing the wound surface area—a vector for infection.
- Material Fatigue: Repeated heating weakens the steel, increasing the risk of breakage.
Today, “disposable” is synonymous with “safety.” It guarantees that every patient receives a pristine, sharp, and certified sterile device.
III. The “Software”: The Core of Aseptic Technique
If the sterile needle is the tool, Aseptic Technique is the operating system. Even the most perfect needle becomes a biohazard the moment it touches a non-sterile surface.
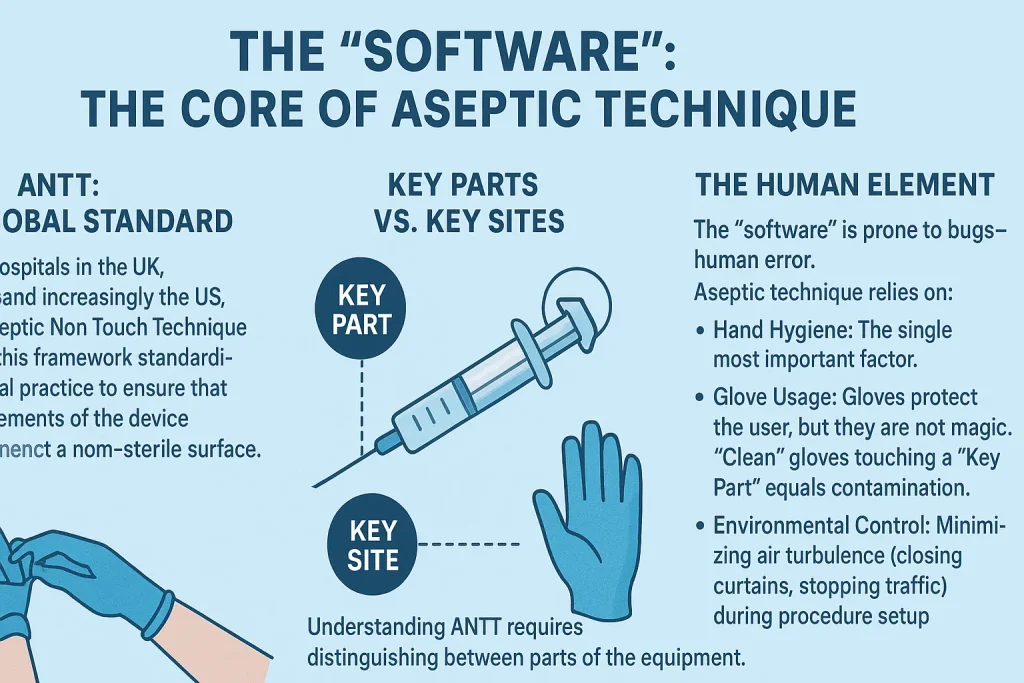
ANTT: The Global Standard
Modern hospitals in the UK, Australia, and increasingly the US, utilize Aseptic Non Touch Technique (ANTT). This framework standardizes clinical practice to ensure that key elements of the device never contact a non-sterile surface.
Key Parts vs. Key Sites
Understanding ANTT requires distinguishing between parts of the equipment and areas on the patient.
Table 2: Defining the “No-Touch” Zones
| Category | Definition | Examples (Must NEVER be touched) |
|---|---|---|
| Key Parts | Parts of the equipment that must remain sterile. If these are contaminated, the patient is directly exposed. | • The needle shaft and bevel• The syringe hub/tip (Luer slip or lock)• The inside of the needle cap• The plunger shaft (in some strict protocols) |
| Key Sites | The area on the patient where the medical device will enter. | • The cleaned skin injection site• The open wound• The catheter insertion site |
The Human Element
The “software” is prone to bugs—human error. Aseptic technique relies on:
- Hand Hygiene: The single most important factor.
- Glove Usage: Gloves protect the user, but they are not magic. “Clean” gloves touching a “Key Part” equals contamination.
- Environmental Control: Minimizing air turbulence (closing curtains, stopping traffic) during procedure setup.
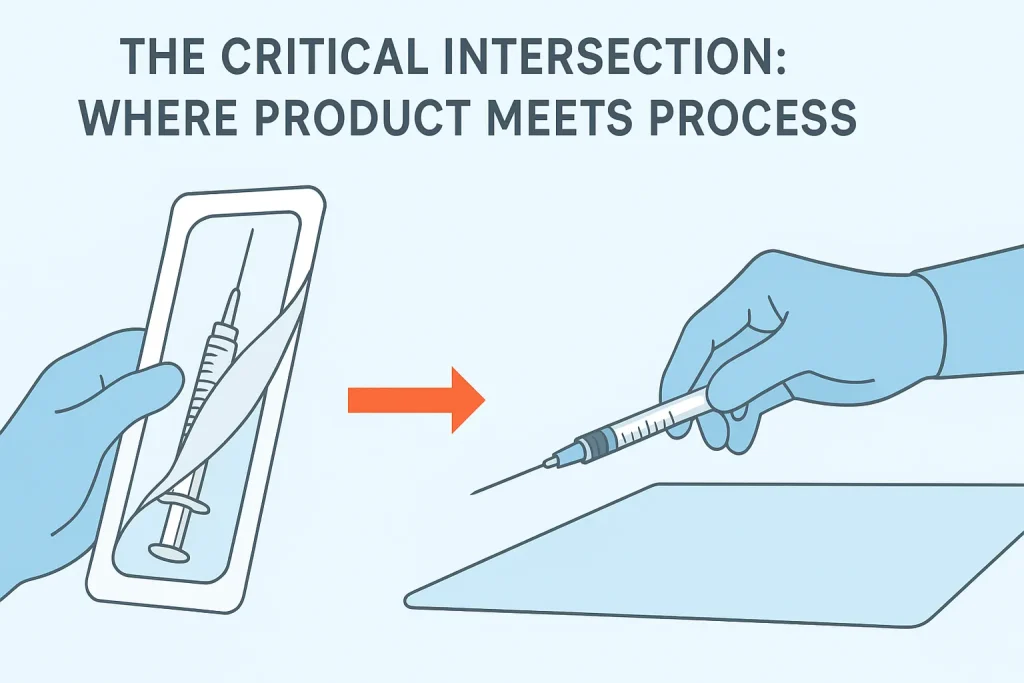
IV. The Critical Intersection: Where Product Meets Process
The moment of highest risk occurs when the product leaves its packaging. This is where manufacturing design and clinical skill intersect.
The “Peel-Pack” Moment
A poorly designed package fights the user. If the paper tears or the seal is too tight, the clinician may struggle, leading to:
- Recoil: The needle jerks out and hits a non-sterile tray.
- Contact: The user’s finger brushes the hub while trying to pry the package open.
Manufacturer’s Role: Leading manufacturers design “peel-pack” systems with distinct separation layers and grip tabs, allowing for a smooth, controlled opening that supports a “non-touch transfer” directly onto a sterile field or onto the syringe.
Safety Mechanisms & Sterility
Retractable insulin needles and safety syringes are often marketed for preventing needlestick injuries (healthcare worker safety), but they also support aseptic technique.
- The Re-Capping Hazard: In traditional injection scenarios, re-capping a needle is a primary cause of accidental contamination (and injury).
- The Safety Solution: By engaging a safety mechanism that covers the needle immediately after withdrawal, the clinician removes the need to manipulate a contaminated sharp, thereby preserving the aseptic integrity of the immediate environment.
Case Study: Escalating Asepsis
- Standard Injection (Clean/Standard Aseptic): Giving a flu shot. The site is cleaned. The sterile needle is attached. Injection is given. Risk is managed via standard “Key Part” protection.
- Hemodialysis (Surgical Aseptic): Inserting a Fistula Needle. Because this needle accesses high-flow blood circulation directly, the risk of sepsis is higher. “Surgical Aseptic” technique is required—sterile gloves, a larger sterile field, and often a mask. Here, the quality of the needle’s protective cap and the ergonomics of the hub are vital to maintain control while wearing bulky sterile gloves.
V. Common Misconceptions & Pitfalls
Even experienced professionals can fall prey to subtle myths that compromise safety.
Myth 1: “The needle is sterile, so I can put it down on a tray.”
- Reality: Unless that tray is covered with a sterile drape and created as a sterile field, it is merely “clean.” A sterile needle placed on a clean tray is now contaminated. It must remain in the package or be capped until the moment of use.
Myth 2: “I’m wearing gloves, so I can touch the needle hub.”
- Reality: Standard examination gloves are clean, not sterile. They come from a box exposed to air. Touching the hub (a Key Part) with a clean glove transfers bacteria from the box/air to the fluid path.
Myth 3: “The package looks fine, so it’s safe.”
- The “False Security”: Micro-punctures can occur if boxes are crushed or stored improperly.
- Best Practice: Users must inspect the seal integrity before peeling. Manufacturers assist this by using color-changing sterilization indicators on the packaging to prove the product has undergone the sterilization process.
Table 3: Technique Comparison
| Technique Level | Goal | Typical Procedure |
|---|---|---|
| Clean Technique | Reduce the number of microorganisms. | Taking blood pressure; routine physical exam. |
| Aseptic Technique (ANTT) | Prevent introduction of microorganisms to key sites. | IV insertion; IM injections; Wound dressing. |
| Surgical Sterile Technique | Eliminate ALL microorganisms from an area. | Major surgery; Central line insertion. |
VI. Conclusion: The Manufacturer’s Commitment
In the battle against infection, the line between success and failure is drawn at the tip of a needle. We must recognize that aseptic technique is the behavior, and the sterile disposable needle is the enabler.
For the clinician, this means adherence to ANTT principles and vigilance regarding “Key Parts.” For the manufacturer, it means an unwavering commitment to ISO standards, robust sterile barrier packaging, and ergonomic designs that minimize handling difficulties.
Healthcare providers and procurement officers must look beyond the price per unit. Choosing high-quality sterile disposable needles involves evaluating the packaging integrity, the clarity of labeling, and the reliability of safety mechanisms.
By investing in superior “hardware,” hospitals reduce the cognitive load on staff, making the “software” of aseptic technique easier to execute, ultimately safeguarding the most important person in the room: the patient.

VII. Authoritative Sources & Further Reading
- World Health Organization (WHO): WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. (Geneva: World Health Organization; 2010).
- Centers for Disease Control and Prevention (CDC): Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care.
- The Association for Safe Aseptic Practice (ASAP): ANTT® Clinical Practice Framework. (www.antt.org)
- International Organization for Standardization (ISO):
- ISO 7864:2016 – Sterile hypodermic needles for single use.
- ISO 11607 – Packaging for terminally sterilized medical devices.
- National Institute for Health and Care Excellence (NICE): Healthcare-associated infections: prevention and control in primary and community care.