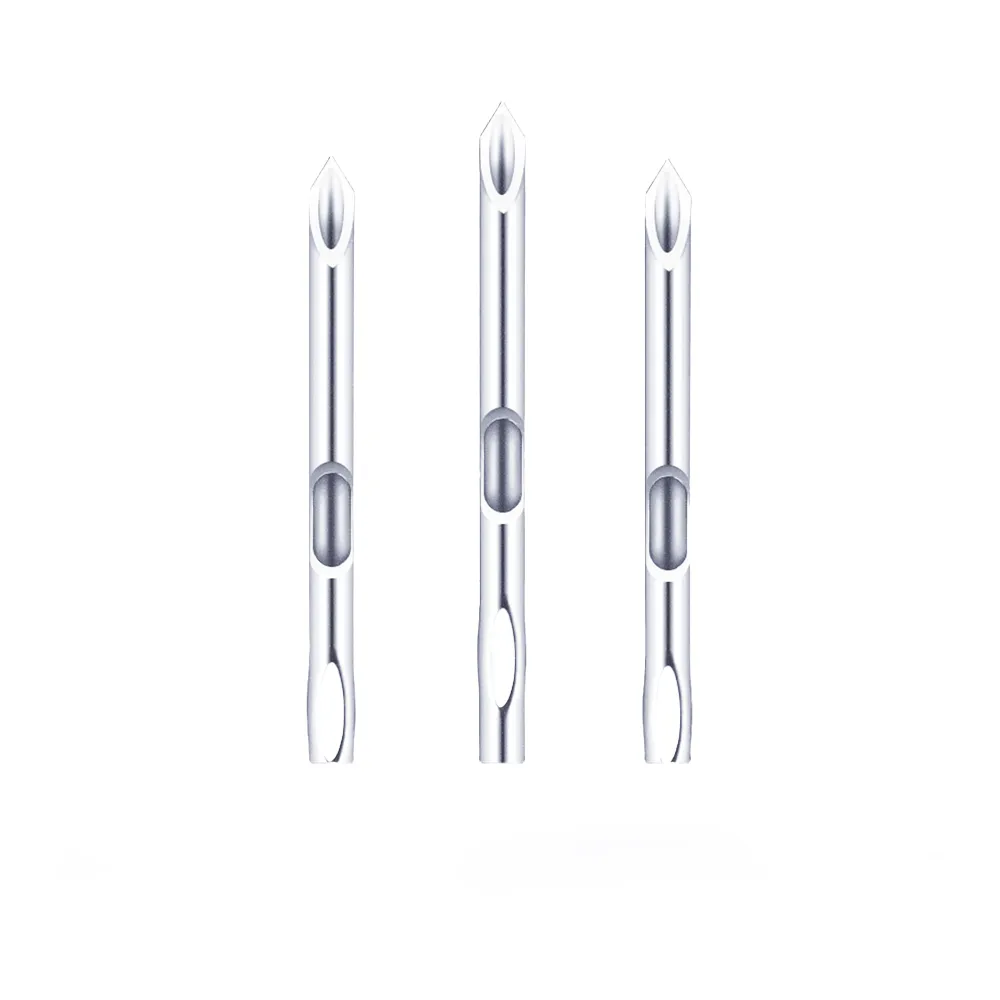
المواصفات الفنية
| المعلمة | المواصفات |
|---|---|
| اسم المنتج | إبرة التداخل (قنية القسطرة الوريدية) |
| المادة - قنية | إبرة طبية من الفولاذ المقاوم للصدأ SUS304 من الدرجة الطبية مع أنبوب قسطرة PTFE / PU |
| نطاق القياس | 14 جرام - 24 جرام (OD: 2.0 مم - 0.6 مم) |
| خيارات طول القسطرة | 19 مم - 45 مم (تفاوت ± 1 مم) |
| تصميم الإكرامية | طرف مشطوف ثلاثي الحواف فائق الحدة؛ ثقب سلس مع تقليل ألم المريض |
| الرؤية بالأشعة السينية | شريط القسطرة المشعة للكشف بالمنظار الفلوري |
| خاصية التحكم في الدم | حجرة التدفق العكسي الاختيارية لتأكيد الوصول الوريدي |
| مادة القسطرة | متوافق حيوياً PTFE/بولي يوريثين متوافق حيوياً، مقاوم للفرنك، خالي من مادة DEHP |
| تصميم المحور | محور شفاف مع مؤشر قياس مرمز بالألوان (حسب ISO 10555-5) |
| خيارات السلامة | بآلية أمان أو بدون آلية أمان (تراجع الإبرة السلبي/النشط) |
| طريقة التعقيم | أكسيد الإيثيلين (EO) المعقم |
| التعبئة والتغليف | عبوات نفطة معقمة بشكل فردي، 50-100 قطعة لكل علبة |
| استخدام واحد | نعم |
| الشهادات | ISO 13485، وعلامة CE، وموافقة إدارة الغذاء والدواء الأمريكية (حسب المنطقة) |
| مدة الصلاحية | 3-5 سنوات في ظروف التخزين الموصى بها |
الإبرة غير المغروسة (قنية القسطرة الوريدية): وصول وعائي متقدم لتوصيل العلاج المستمر.
الإبرة الداخلية الغرض السريري
توفر إبرنا التي يتم إدخالها في الأوعية الدموية وصولًا آمنًا وطويل الأمد لإعطاء السوائل الوريدية وتوصيل الأدوية وأخذ عينات الدم. وقد صُممت هذه الإبر لتوصيل السوائل الوريدية لمدة تصل إلى 96 ساعة، وهي مصممة لتوصيل السوائل الوريدية لمدة تصل إلى 96 ساعة، وتقلل من تكرار عمليات الثقب في الرعاية الحرجة وطب الأورام وطب الأطفال.
مواصفات المنتج:
1. نوع المنتج: نوع Y، النوع المستقيم، نوع الأمان، نوع التراجع، المسافة البادئة.
2-المواصفات: 10G-28G
3.OD: 3.4 مم - 0.36 مم
المزايا الرئيسية
تقنية الثقب الدقيق
- يتيح الطرف ثلاثي الحواف المشحوذ بالليزر (زاوية طحن بزاوية 14 درجة) اختراقًا سلسًا للأنسجة مع قوة إدخال أقل بمقدار 30% مقارنة بالإبر القياسية.
- تقلل حافة القطع الحادة للغاية من صدمة الأوعية الدموية وانزعاج المريض (تم التحقق من صحة درجات الألم في VAS ≤2/10).
تعزيز السلامة والرؤية المعززة
- أجنحة قابلة للكشف بالأشعة السينية وخط القسطرة لتأكيد الموضع تحت التنظير الفلوري.
- تتميز الإصدارات المصممة خصيصاً للسلامة:
- سحب الإبرة تلقائيًا عند السحب (نوع السحب)
- تنشيط القنية الحادة (نوع الأمان)
- التفعيل بيد واحدة (متوافق مع إدارة السلامة والصحة المهنيتين)
الابتكار في المواد
- قنية: من الفولاذ المقاوم للصدأ 316L من الدرجة الطبية (OD: 0.36-3.4 مم) مع صقل داخلي كهربائي
- القسطرة: DEHP-البولي يوريثين الخالي من البولي يوريثين (تصميم جدار رقيق يحافظ على معدلات تدفق تصل إلى 300 مل/دقيقة)
- المحور: بولي كربونات متوافقة مع التصوير المقطعي المحوسب/التصوير بالرنين المغناطيسي مع قفل لوير
التطبيقات السريرية
- التسريب المستمر للعلاج الكيميائي
- إنعاش السوائل في حالات الطوارئ
- العلاج بالمضادات الحيوية طويل الأمد
- الوصول الوريدي لحديثي الولادة/الأطفال حديثي الولادة
الامتثال للجودة
- تشعيع أشعة غاما EO المعقمة | ISO 10555-5 | FDA 510(k) K230755
- محاور مرمزة بالألوان لتحديد المقياس
- التخصيص: طول أنبوب التمديد (15-50 سم)، مشابك أمان سلبية