Technical Specifications
| Parameter | Specification |
|---|---|
| Product Name | Indwelling Needle (IV Catheter Cannula) |
| Material – Cannula | Medical-grade SUS304 stainless steel needle with PTFE / PU catheter tube |
| Gauge Range | 14G – 24G (OD: 2.0 mm – 0.6 mm) |
| Catheter Length Options | 19 mm – 45 mm (±1 mm tolerance) |
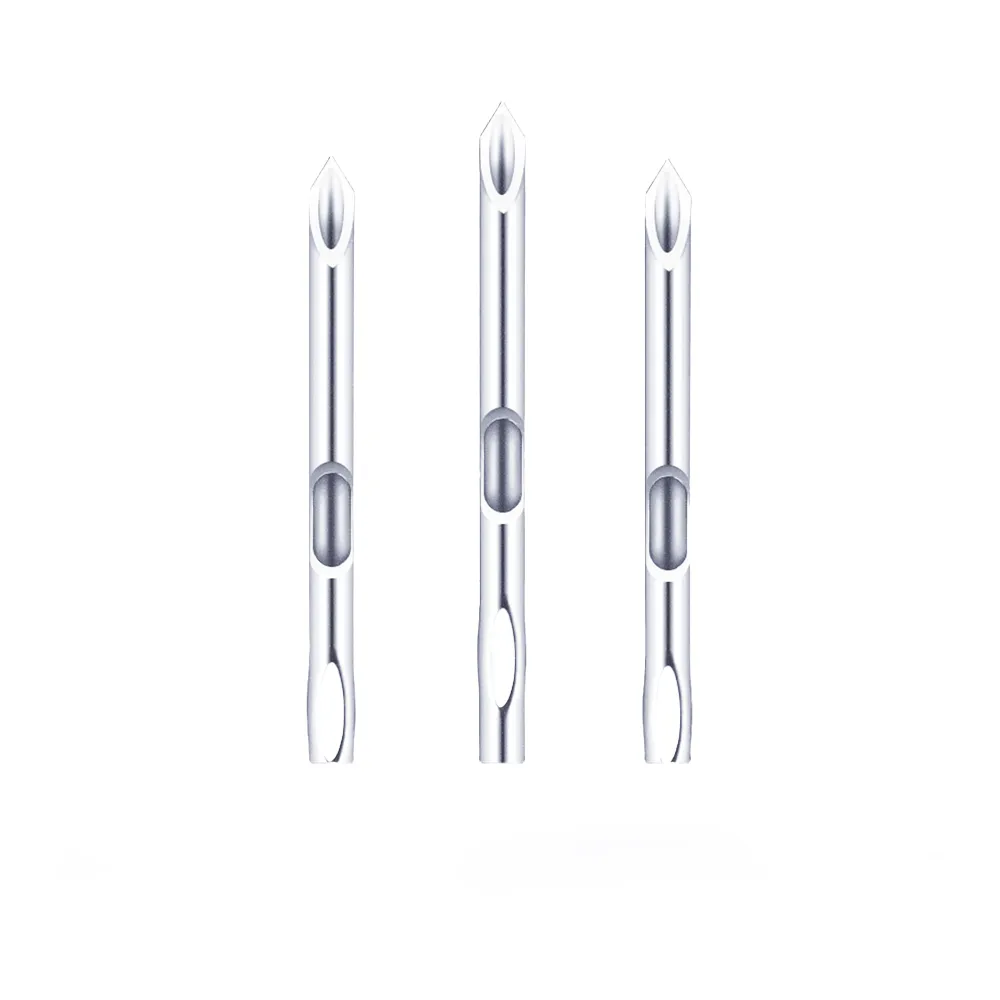
| Tip Design | Tri-beveled ultra-sharp tip; smooth puncture with reduced patient pain |
| X-ray Visibility | Radiopaque catheter strip for fluoroscopic detection |
| Blood Control Feature | Optional backflow chamber for venous access confirmation |
| Catheter Material | Biocompatible PTFE / Polyurethane, kink-resistant, DEHP-free |
| Hub Design | Transparent hub with color-coded gauge indicator (per ISO 10555-5) |
| Safety Options | With or without safety mechanism (passive / active needle retraction) |
| Sterilization Method | Ethylene Oxide (EO) sterilized |
| Packaging | Individually sterile blister packs, 50–100 pcs per box |
| Single-Use | Yes |
| Certifications | ISO 13485, CE marking, FDA approval (region dependent) |
| Shelf Life | 3–5 years under recommended storage conditions |
Indwelling Needle (IV Catheter Cannula): Advanced Vascular Access for Sustained Therapy Delivery.
Indwelling Needle Clinical Purpose
Our indwelling needles provide secure, long-term vascular access for intravenous fluid administration, medication delivery, and blood sampling. Engineered for indwelling durations up to 96 hours, they minimize repeated punctures in critical care, oncology, and pediatric settings.
Product Specification:
1. Product type: Y type, straight type, safety type, retraction type, indentation.
2.Specification:10G-28G
3.OD:3.4mm-0.36mm
Key Advantages
Precision Puncture Technology
– Laser-honed triple-bevel tip (14° grind angle) enables smooth tissue penetration with 30% less insertion force vs. standard needles.
– Ultra-sharp cutting edge reduces vessel trauma and patient discomfort (validated by VAS pain scores ≤2/10).
Enhanced Safety & Visibility
– Radiopaque X-ray detectable wings and catheter line for position confirmation under fluoroscopy.
– Safety-engineered versions feature:
– Automatic needle retraction upon withdrawal (retraction type)
– Blunt cannula activation (safety type)
– One-handed activation (OSHA-compliant)
Material Innovation
– Cannula: Medical-grade 316L stainless steel (OD: 0.36–3.4mm) with electropolished interior
– Catheter: DEHP-free polyurethane (thin-wall design maintains flow rates up to 300mL/min)
– Hub: CT/MRI-compatible polycarbonate with Luer-Lock
Clinical Applications
– Continuous chemotherapy infusion
– Emergency fluid resuscitation
– Long-term antibiotic therapy
– Neonatal/Pediatric IV access
Quality Compliance
– Sterile EO gamma irradiation | ISO 10555-5 | FDA 510(k) K230755
– Color-coded hubs for gauge identification
– Customization: Extension tube length (15–50cm), passive safety clips